Hi Pietro,
you can activate and use the BIAS electrode while using ThinkPulse sensors, we do it on a regular basis and it works fine. BIAS usually makes the signal more stable, limits offset and 50/60Hz noise. There might be specific situations where you do not want BIAS activated but ThinkPulse sensors can definitely be used with BIAS activated.
Best,
Julien
Over on the other recent thread link below, @lucasbaldezzari remarks that he can use his SSVEP BCI acceptably with his gel based (wet) cap system. But when he uses DRY electrodes (either passive dry, or with your active dry system), the results are noisy and not reliably producing the SSVEP results he expects. Do you have any insights on how he could adjust scaling or other parameters to make your active dry system equivalent with wet results?
I am guessing that many users and potential users of active dry systems, are assuming that an active dry system would perform similarly to a wet system. And other very expensive manufacturers of active dry systems, such as Cognionics or g.tec SAHARA, do claim their systems are as good as wet.
@pg30 I have done some pretty heavy testing with the ThinkPulse Active Electrodes and MarkIV. The next major version of the GUI will have a mode that improves the user experience for this product in the OpenBCI GUI. You can see additional info on this and track the progress on the GitHub issue I have made to address this.
All in all, they work great! I have been using this for a few months. The combs are a bit shorter than the long-haired comb electrodes, so moving hair aside is important. The biggest area for improvement at this time is pre-setting the Cyton channel settings automatically in the GUI, and updating the documentation, which will be resolved by GitHub issue.
I read that the BIAS should be turned off in the GUI. Does that mean that there will be no ground/bias electrode?
I have tried with this turned on and off. I will try again when resolving this issue in the next month. At this time, I recommend dropping the gains and experimenting a bit. Feel free to tag me here on this forum post or on the GitHub issue to share your results if you start using them in the meantime.
Thank @wjcroft for mentioning me. Like you said, I have no SSVEPs using dry passive electrodes and neither active.
I'm using Brainflow in order to make a BCI using SSVEPs. I have been trying to get SSVEPs using Ganglion Board and Cyton Board with passive and active electrodes. When I tested with the Cyton and the Active electrodes (both with ultracortex), I disconnected from the BIAS all the channels I didn't use, beside, I ran the experiment with gains x1, x2, x4 and x8 when I used them. On the other hand, with the Cyton and the Passive Electrodes I use the gain in x24 (and only use the channel 1 and 2, but channels from 3 to 8 were disconnected from the bias). I tried using the Ganglion board, not only with the passive electrodes, but also one think pulse. But no SSVEPs.
I need to mention that when I use the GUI in order to see the EEG signals (coming from Ganglion, or Cyton with passive or active electrodes), the signal looks fine enough (I can see huge alpha waves, for example), but when I run my experiment, I can not see the SSVEPs (but I am pretty sure that there must be SSVEPs because when I use the gel cap for the same experiment I see them). So, I don't know what's going on, it's weird.
Trust me, I ran several experiments so I was thinking that perhaps the problem are the dry electrodes, but, why? Or perhaps there is something that I'm doing wrong.
I will let you know if I found some results.
Regards,
Hi everyone,
it is possible to use dry electrodes for SSVEP. Hard to tell what's wrong here: the most common issue is that the hardware parameters are not correctly applied, as @retiutut mentioned it is sometimes tricky, especially if you stream the EEG flow to a third party software. The modifications of hardware parameters like gain, deactivation of a channel or bias are sometimes not correctly/totally implemented and validated in generic software even when Cyton/Ganglion are officially supported. @lucasbaldezzari is the signal you're looking at in the GUI the same signal that is processed ? Another thing that could possibly explain your problem, the default parameters that work for a SSVEP BCI with gel electrodes might not work directly when you swap electrodes to dry ones. Basically, dry electrodes tend to be a little more sensitive to noise if the skin is dry, if the pressure/contact is not right, if the hair are not combed away, if there is excessive EM noise from mains, etc... In comparison papers like the ones @wjcroft mentioned from some manufacturers, the comparison tests are usually performed in close to ideal conditions and there is basically no difference then between dry and gel (especially if the electrodes are pointy/rigid/metal/semi dry). If you are not in ideal conditions, you might get a little more noise and then you might have to adjust the SSVEP parameters, like maybe increasing the duration of acquisition before identifying the right frequency, adjust the filters. A preliminary step might be to assess the evoked potential response in the FFT first and figure out what parameters should be slightly adjusted. We got great results using C-VEP with ThinkPulse for spelling (using OpenBCI GUI and LSL) so I see no reason why you could not succeed with dry electrodes. Best, Julien
@lucasbaldezzari said:
...
I need to mention that when I use the GUI in order to see the EEG signals (coming from Ganglion, or Cyton with passive or active electrodes), the signal looks fine enough (I can see huge alpha waves, for example), but when I run my experiment, I can not see the SSVEPs (but I am pretty sure that there must be SSVEPs because when I use the gel cap for the same experiment I see them). So, I don't know what's going on, it's weird.
If the SSVEP peak in the FFT is obvious and strong, and your detection / classification algorithm / filter is not matching it, then it seems to imply some adjustment is needed in your detection criteria.
@lucasbaldezzari is the signal you're looking at in the GUI the same signal that is processed ?
I hope so! But sometimes I think for some reason the signals perhaps are different, it has no sense because I'm using the Brainflow documentation and Open BCI documentation and I have configured the Ganglion or the Cyton boards in the same way that the GUI (the channels disconnection, gains) but perhaps I'm doing something wrong.
If you are not in ideal conditions, you might get a little more noise and then you might have to adjust the SSVEP parameters, like maybe increasing the duration of acquisition before identifying the right frequency, or adjusting the filters.
I did it what you mentioned, I increase the stimulation time, more LEDs for stimuli, changed the LEDs color, I use stimuli in a screen, I increase the number of averaged trials, but for dry electrodes, I haven't SSVEPs, but for gel cap, everything is fine. So, it is weird. It should be something of hardware configuration.
If the SSVEP peak in the FFT is obvious and strong, and your detection / classification algorithm / filter is not matching it, then it seems to imply some adjustment is needed in your detection criteria.
I use this comment to show you some pics (I accidentally clicked "Post Comment", sorry.
All the experiments were performed using one Blue LEDs with a blinking frequency. The frequency was made using an Arduino with a square signal. I performed a 3 trial averaged an 4 seconds of stimulation for each trial and 2 seconds for rest time between trials. I only use 2 channels (O1 and O2). I applied a notch filter in 50Hz and a Bandpass Butterworth filter between 5 and 30Hz.
Next image shows the Fourier Spectrum for the 8.5Hz stimulus. You can see a 8.5 peak. This is for channel O1 only and gel cap electrodes.
Next image is the PSD for Welch method for the 9Hz Stimuli for O1 and O2 channels averaged and gel cap electrodes.
Next image is the Fourier spectrum for the 7Hz Stimuli for channel O1 only and gel cap electrodes. There are one peak at 7Hz and other in 14Hz (personally, I have noticed that at 7hz the first harmonic almost always exceeds the central frequency in amplitude, I think this is reported in the literature).
Finally, this is the Fourier spectrum for 7Hz stimuli but using Dry electrodes. This spectrum corresponds to the 7Hz Stimuli for channel O1 only. This corresponds for one of the three averaged trials, but if I show you any of the others averaged trials, the results are similar, I mean, the spectrum is very noisy and there aren't peaks either in the center frequency nor in the first harmonic.
I have a lot of more signals using gel cap electrodes and the system perform very well. I tried SVM, LDA, CNN, Logistic Regression like classification methods and all of them perform well when I use gel cap electrodes and the Fourier spectrom or the PSD from Welch's method like feature vectors.
All this work is because I am directing a BCI competition where my students will control a robotic vehicle through a race with obstacles using SSVEPs. The race will be the December 17. I will public the github repository when we finish, maybe it will be useful to you.
Hi Lucas,
thanks for images and for the explanations. Your experiment looks great and indeed you can see a very nice peak with the gel electrodes. I'm confident you can achieve similar results with the dry electrodes but it might require some adjustments. I do not know if this is the right place to discuss the SSVEP protocole here, and I'm not an expert in this but my first impressions for the dry electrode spectrum are 1/ your 7Hz peak is still here, with similar amplitude compared to previous spectrum and this is a local maximum. It looks like the 14Hz is here too with similar amplitude. The problem is more that other peaks are present with pretty high amplitudes too (9/10Hz, 19/20) and especially an extra 16Hz peak that is over 0.8 microV and higher than others, not present in the other spectrums. Is there anything around you that could explain this extra peak ? Since I believe your experiments were done in a similar environment with gel or dry electrodes, it looks like you are catching more EM noise with the dry electrodes hence the noisier spectrum. I would suggest to try to reproduce the experiment in a very non noisy location and also to make sure nothing around in your setup could produce some noise (computer, electronics, chargers, printer,...). Also it is always good to make sure you have a good contact of the sensors on the scalp, and also to try deactivating the potential other channels that do not show good contact/good signal, they could contaminate the good signal. Good luck with that and let us know how it goes !
Best,
Julien
What I don't understand about the posted graphs is the vertical scale. Is there a mistake? All these values are below 1 microvolt. EEG less than a microvolt is generally considered mostly noise. What FFT vertical scale peaks do you see when looking at this in the GUI? I would expect VEP peaks at least a few microvolts, probably more.
Hello guys, how are you?
Thank you @julienConscious. I will answer you.
Your experiment looks great and indeed you can see a very nice peak with the gel electrodes. I'm confident you can achieve similar results with the dry electrodes but it might require some adjustments
Thanks for your comment, I will try to get the same results using dry electrodes.
and I'm not an expert in this but my first impressions for the dry electrode spectrum are 1/ your 7Hz peak is still here, with similar amplitude compared to previous spectrum and this is a local maximum. It looks like the 14Hz is here too with similar amplitude.
Yeap, perhaps there are some SSVEPs information, but I'm not sure. I think it is only EEG activity.
Since I believe your experiments were done in a similar environment with gel or dry electrodes, it looks like you are catching more EM noise with the dry electrodes hence the noisier spectrum.
Yes, you're right. But I will pay more attention and try again.
Also it is always good to make sure you have a good contact of the sensors on the scalp, and also to try deactivating the potential other channels that do not show good contact/good signal, they could contaminate the good signal.
Great, I will do this.
Good luck with that and let us know how it goes !
Thank you @julienConscious, I will share the results with you as soon as possible.
What I don't understand about the posted graphs is the vertical scale. Is there a mistake? All these values are below 1 microvolt. EEG less than a microvolt is generally considered mostly noise. What FFT vertical scale peaks do you see when looking at this in the GUI?
Yeap, it is weird. In the GUI app I can see more than 1uV. Please refer to the images below where you can see from left to right, the EEG for O1 and O2 averaged channels (filtered using a notch in 50Hz and a band pass between 5Hz-30Hz), O1 and O2. Then you can see the FFT and the PSD using the Welch method. Those images are for the 8.5Hz stimulus. Perhaps I'm implementing something wrong in my codification and that's the reason for very low amplitudes. What do you think?
Hi Lucas,
so I was curious, we made some quick experiments.
Here is the spectrum for O2 with a blinking at 7Hz:
and the spectrum still for 02 with a blinking at 11Hz:
Both recordings were done using the ThinkPulse active sensors over 20s (I did not try a shorter time), simple FFT, no Welch. We can see the expected peaks. What I noticed is how important it is to have a small 50Hz (or 60Hz) interference peak. It does not work well if the 50Hz interference is too high: dry sensors tend to catch more of this interference compared to wet sensors in the same conditions which could explain the problem in your experimentation. So I would suggest checking the unfiltered FFT in the OpenBCI GUI live and check the amplitude of the 50Hz peak, try to find a spot where it is as low as possible and do the experiment again. Keep us posted!
Best,
Julien
Hello @julienConscious, how are you? Thank you so much for your time, I appreciate it.
Your graphs look very nice. Luckily last friday my students made experiments, they controlled robotics vehicles using bci. For this, they use gel cap electrodes and gold cup electrodes -and Ganglion boards-. Everything was fine. I share with you a graph for the power spectral density using the gel cap -with three averaged trials- and some pics about the race.
Thank you for your support @julienConscious and @wjcroft. I will be around the forum next year, for sure!. Have a nice end of year.
Hi @wjcroft and @julienConscious,
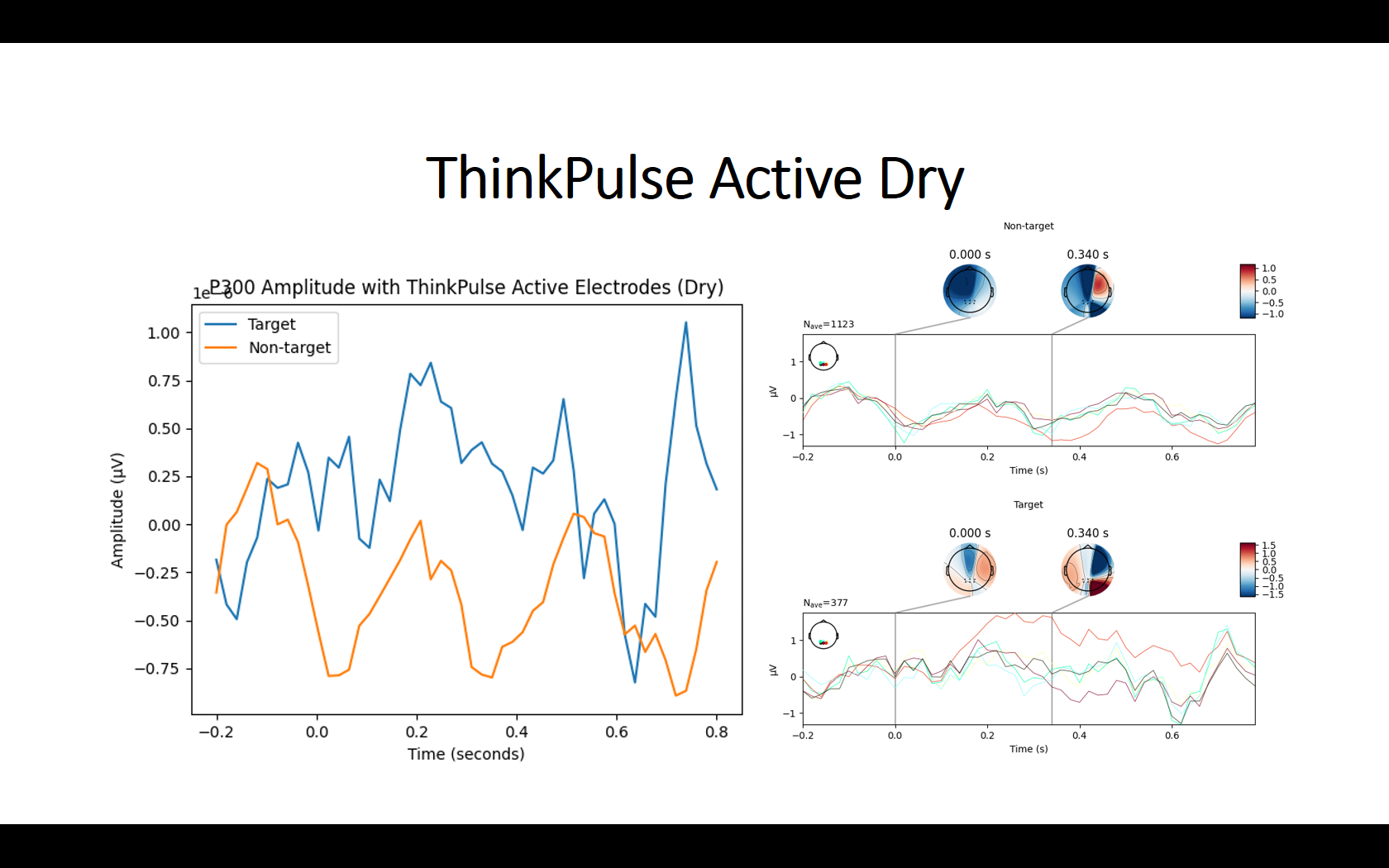
I have recently purchased the ThinkPulse active electrodes and the OpenBCI Cyton + Ultracortex M4 and am trying to run a P300 experiment on it. However, I can’t seem to detect any P300 signal when using these active electrodes, which is why I’m hoping I can get some advice here.
To provide some context, I set up the headset with 8 electrodes (Fp1, FP2, PO3, POz, PO4, O1, Oz, O2) and wired everything according to the ThinkPulse videos (with ear clips at SRB2 and BIAS). I’m using the OpenBCI GUI to send the data via the LSL Networking module. As a sanity check, I opened the OpenBCI GUI visualizer to inspect the timeseries data. Even without disconnecting the BIAS earclip or changing the hardware settings, the signals seemed fine – I could see eye blinks, teeth clenching, and alpha waves from eyes closed recordings (see screenshots below).
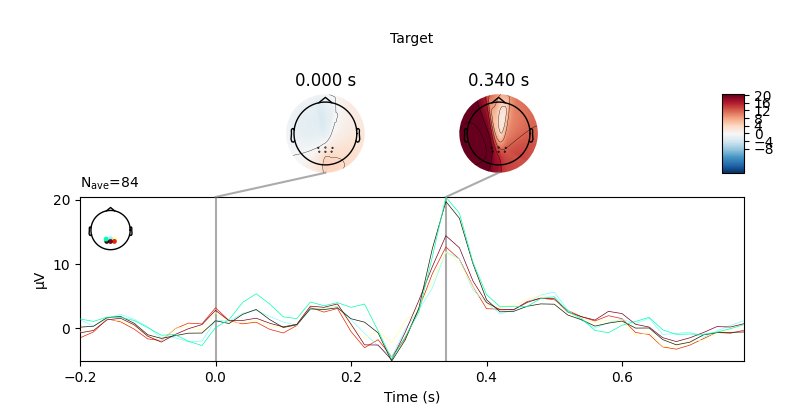
I then tried to collect data while doing a simple visual P300 experiment where I randomly flash a 4x4 grid. I collected around 15 minutes worth of data, which contains about 420 trials of flashing of the target button. To preprocess the data, I applied a bandpass filter from 1-25 Hz and resampled the signal to 50 Hz. I then averaged all the target trials to one grand average ERP (without the Fp1 and FP2 electrodes) to visualize the P300. However, I couldn’t see any noticeable ERP with the ThinkPulse electrodes. To make sure it wasn’t a problem with the experiment setup itself, I repeated the exact same experiment using a research-grade wet EEG cap (gel), and the P300 signal was very clear with an amplitude of 20 uV (see screenshots below).
To rule out it was an issue with not having enough trials, I tried averaging only 3 minutes worth of data for the wet EEG cap (only 84 trials) and I still saw a P300 with an amplitude of 20 uV. Even though dry systems are noisier, it seems unlikely that I couldn’t notice the signal after averaging 377 trials using the ThinkPulse electrodes.
I was told that Conscious Labs had success measuring the P300 wave with the ThinkPulse active electrodes, so I’m confused why I’m not getting similar results. I’m not sure what could be going wrong here and would appreciate your help. I really hope I can get these electrodes to work. If they do, I definitely plan on buying more sets of them. Sorry for the long post, but please let me know if there are other details I can provide to help clarify things.
Hi @Jason,
thanks for your message and the detailed description of your experiment. We indeed have successful P300 experiments protocoles with ThinkPulse, sorry to hear it did not work for you. What you did for verification makes complete sense. Hard to tell what goes wrong when you use the ThinkPulse. It looks indeed like you have a very clean physiological EEG signal with the ThinkPulse setup. Even if the recordings were a bit noisier, the high number of repetitions of the stimuli should definitely increase the signal over noise ratio and show a P300 wave. A few questions:
did you use the same locations for the sensors between wet and dry?
did you use LSL in both cases (to rule out a problem in the synchronization between display and EEG -> there is a peak at 700ms that is weird)?
why is the amplitude for the dry electrode around 10 microV vs amplitude for TP electrodes around 1 microV in the P300 analysis figures whereas in the OpenBCI GUI we can see that the scale is 200microV and that the signal amplitude is closer to 10 microV probably ?
did you check that the screen display is not causing artifacts for TP ?
have you checked channel per channel instead of grand average?
did you reject outliers epochs ? It's pretty easy over a 15 min recording to move a bit or create artifacts with face muscles, they tend to impact the signal a lot with dry sensors and can result in big artefacts. Standard averaging is not robust to outliers. Moreover, if you do have a rejection filter based on amplitude, it might not work properly if the amplitude of the TP is inconsistent (and smaller) with what you see in the OpenBCI GUI.
Best,
Julien
Sorry for my English and sorry .. for the question, if possible!
About 18 months ago in this forum I posed the problem of ThinkPulse sensors+Ultracortex Mark IV + Cyton not working while the Cap with wet electrodes + Cyton worked very well (BCI P300 speller application).
In the last few days I was about to try the system again (ThinkPulse sensors+Ultracortex Mark IV + Cyton), but first I wanted to have a look on the Forum to see if there was anything new. Well, over the past 18 months, I have read more reports of malfunctions (both: SSVEP and P300).
(one: https://openbci.com/forum/index.php?p=/discussion/comment/17679#Comment_17679)
I thought: perhaps because there are only reports of problems on the Forum.
So I had an idea: is it possible to ask if there are any Openbci users who are working well with P300 speller applications and with ThinkPulse sensors+Ultracortex Mark IV + Cyton?
At the moment I read that only JulienConscius, by Paris, says that ThinkPulse sensors work well ... . He produces them... . (and I don't think he works with Ultracortex Mark IV + Cyton).
Also Retiutut wrote that he was working "great" (dicembre 2021). But is it so? I dont'read any about his work in 2022.
My question is: is it possible to ask if are there Openbci users who work well with ThinkPulse sensors+Ultracortex Mark IV + Cyton?
At the moment, it is as if we have a dozen ratings for a product sold on Amazon, all at a minimum.
I hope that Openbci will sell wet electrodes of the same quality as its other products (Cyton, cap with dry electrodes, etc...) in the near future.
Thank you!
Etc
I merged your new thread into this existing thread on the same subject. It is better to group related thread subject areas. That way, previous commenters see your new comment via email, because they are 'subscribed' to the thread. In particular, @julienConscious, the ThinkPulse developer will see your comment. Thanks.
In looking through this thread right now, I do not see your past comment on Thinkpulse. However I do see that you had a comment on the saline electrode cap, here:
Hi everyone,
thanks William as usual for organizing the discussions in the forum and for including the people that might be interested in participating in the discussion.
Thanks Etc for your interest in BCI and for spending time testing our tech. Recording P300 waves can be tricky with TP+UltraCortex Mark IV+Cyton. I'm happy to help if you want to share results here or using DM: we can organize a call and see what's wrong (and post results in the forum later to help others). Good luck with your BCI experiments and I sincerely hope you will succeed in reaching your goals.
Best,
Julien
Hi everyone,
I got the P300 paradigm to work with the TP electrodes after all, for both offline and online trials. Here are some plots if you're interested. They show the averaged ERP response for nontarget and target trials for the offline and online trials respectively.
While this did work, I want to note that the sensors pick up noise very easily. I had to keep my head very still when doing this and even so, I can see high frequency bursts being picked up by the sensors every 10 seconds or so. Regardless, it didn't affect my results presumably because the bandpass filter I applied suppressed these artefacts.
Another disclaimer, this also isn't reliable for me 100% of the time. Sometimes I would not see any significant P300 response at all when I'm doing this in another room for example. I wonder if that has something to do the WiFi not being great there (since the OpenBCI data is transmitted over WiFi).
Anyhow, just wanted to share my results with the community. Please feel free to reach out to me if you have questions.
Thanks,
Jason
(Sorry for my very bad English) @wjcroft, Dear Williams,
thank you for your organizing the discussion! (I wrote some comments about ThinkPulse in this forum inside a discussion about “issues with my 2 Cyton dongles / speller apps [resolved]))";
@JulienConscious,
after reading about ten post in these months with ONLY description of problems by users of TP+UltraCortex Mark IV+Cyton, I began to think that there are problems in this solution (Tp+UltraCortex Mark IV+Cyton). Before to try again to work with Tp+UltraCortex Mark IV+Cyton and before to spend a lot of time, I asked if is there an happy user of TP+UltraCortex Mark IV+Cyton. You in the past were very gentle to give suggestions to everybody: but, after your suggestions I didn't read about anyone that worked well
Now, I say thank you to @jason!
I read, after my question, for the first time that there is one happy (with some problems...) user!
Now I would ask to Jason what paremeters did he use; Have you some other suggestions?
I hope that there are other happy users of TP + UltraCortex Mark IV+Cyton to share the results.
In this manner we can read not only problems but also solutions!
Etc
ps Remember: my English is bad...
Hi @etc,
I just used 5 TP electrodes (PO3, POz, PO4, O1, O2) with reference (TP electrode) at Fz and ground (OpenBCI passive flat electrode) at Fpz. Julien actually recommended that the ground electrode should be a passive electrode, while the reference can be an active one. In the OpenBCI GUI, I kept all the hardware settings at the default, except I needed to turn off all the channels that are not being used (by setting "Input Type" to "Shorted"). Other than that, there were no other special hardware settings I needed to tweak.
Hope that helps.
Jason
I just left them as the default. Julien mentioned that you could lower them if the amplitude of the signal is too high, but leaving it on the default settings worked for me.
Thank you!
Last question: what tipe of electrode-Openbci did you use for the ground at Fpz? One of the electrodes of UltraCortex Mark IV?
Thank you very much! You were very gentle!!!
I just used the passive flat ones that came with the kit since I'm putting it at the forehead with no hair. I suspect the dry comb electrodes would work too. The ground electrode just needs to be passive I believe.
hi, I'm Anto , I tested THINKPULSE™ ACTIVE ELECTRODE KIT (Active Dry Electrodes) with our EEG acquisition System , but it's not Picking Signals if I connect Directly on Subject .
I supplied power supply of about +2.5V and -2.5. If I Give 100uV Sine Wave then its Working . And For Ground and Reference electrode I used Gel PAD Electrodes (Surface mount Electrode) and For Active I connected the THINKPULSE™ ACTIVE ELECTRODE via Gel Pad (Gel Pad Electrode is connected on Subject, From Gel pad to Spikes of Dry electrode I Used Crocodile Clip Connectors) then Its Picking the Signal(But only with Gel PAD instead of Directly placed on Subject Head).
I think Circuit Connection and Power Supply , All are okay because Its picking 100uV Sine Wave and Bio-signals only via Gel PAD but only not working if I connect directly on Subject .
Can Any one Help me to Solve this . Thank You.
I merged your new thread into this existing thread on Thinkpulse. Since they were designed for Cyton, I'm not clear that they would work with other "EEG acquisition systems". @julienConscious would know better.
Note the previous comments in this thread, the reference can be either ACTIVE or PASSIVE. You are currently using a passive gel electrode for the reference, you might try as active.
@anto said:
... And For Ground and Reference electrode I used Gel PAD Electrodes (Surface mount Electrode)
But more importantly note that your below comment, does not appear to be 'compatible' with normal Thinkpulse instructions:
and For Active I connected the THINKPULSE™ ACTIVE ELECTRODE via Gel Pad (Gel Pad Electrode is connected on Subject, From Gel pad to Spikes of Dry electrode I Used Crocodile Clip Connectors) then Its Picking the Signal(But only with Gel PAD instead of Directly placed on Subject Head).
The Thinkpulse conductive rubber comb should be in direct contact with the skin. There is no need to connect these combs to gel electrodes, in fact that may confuse the active electronics, most likely. Not sure why you are doing this, the whole idea of Thinkpulse is that it is a dry system. It's fine to connect Ground (or Reference) to a gel electrode.
Comments
Hi @pg30, I merged your question regarding Thinkpulse and Bias, into this existing Q&A thread on Thinkpulse.
The developer, @JulienConscious , may see your question and answer. Or you can also click his blue username, and use the Message button.
William
Hi Pietro,
you can activate and use the BIAS electrode while using ThinkPulse sensors, we do it on a regular basis and it works fine. BIAS usually makes the signal more stable, limits offset and 50/60Hz noise. There might be specific situations where you do not want BIAS activated but ThinkPulse sensors can definitely be used with BIAS activated.
Best,
Julien
Sounds good, thanks for the clarification.
@julienConscious, hi.
Over on the other recent thread link below, @lucasbaldezzari remarks that he can use his SSVEP BCI acceptably with his gel based (wet) cap system. But when he uses DRY electrodes (either passive dry, or with your active dry system), the results are noisy and not reliably producing the SSVEP results he expects. Do you have any insights on how he could adjust scaling or other parameters to make your active dry system equivalent with wet results?
https://openbci.com/forum/index.php?p=/discussion/3191/no-ssveps-using-dry-passive-and-active-electrodes
I am guessing that many users and potential users of active dry systems, are assuming that an active dry system would perform similarly to a wet system. And other very expensive manufacturers of active dry systems, such as Cognionics or g.tec SAHARA, do claim their systems are as good as wet.
https://www.cgxsystems.com/publications
https://www.gtec.at/product/g-sahara-hybrid-eeg-electrodes/
Regards, William
@pg30 I have done some pretty heavy testing with the ThinkPulse Active Electrodes and MarkIV. The next major version of the GUI will have a mode that improves the user experience for this product in the OpenBCI GUI. You can see additional info on this and track the progress on the GitHub issue I have made to address this.
All in all, they work great! I have been using this for a few months. The combs are a bit shorter than the long-haired comb electrodes, so moving hair aside is important. The biggest area for improvement at this time is pre-setting the Cyton channel settings automatically in the GUI, and updating the documentation, which will be resolved by GitHub issue.
https://github.com/OpenBCI/OpenBCI_GUI/issues/999
I have tried with this turned on and off. I will try again when resolving this issue in the next month. At this time, I recommend dropping the gains and experimenting a bit. Feel free to tag me here on this forum post or on the GitHub issue to share your results if you start using them in the meantime.
Take Care,
RW
Hello everyone! How are you?
Thank @wjcroft for mentioning me. Like you said, I have no SSVEPs using dry passive electrodes and neither active.
I'm using Brainflow in order to make a BCI using SSVEPs. I have been trying to get SSVEPs using Ganglion Board and Cyton Board with passive and active electrodes. When I tested with the Cyton and the Active electrodes (both with ultracortex), I disconnected from the BIAS all the channels I didn't use, beside, I ran the experiment with gains x1, x2, x4 and x8 when I used them. On the other hand, with the Cyton and the Passive Electrodes I use the gain in x24 (and only use the channel 1 and 2, but channels from 3 to 8 were disconnected from the bias). I tried using the Ganglion board, not only with the passive electrodes, but also one think pulse. But no SSVEPs.
I need to mention that when I use the GUI in order to see the EEG signals (coming from Ganglion, or Cyton with passive or active electrodes), the signal looks fine enough (I can see huge alpha waves, for example), but when I run my experiment, I can not see the SSVEPs (but I am pretty sure that there must be SSVEPs because when I use the gel cap for the same experiment I see them). So, I don't know what's going on, it's weird.
Trust me, I ran several experiments so I was thinking that perhaps the problem are the dry electrodes, but, why? Or perhaps there is something that I'm doing wrong.
I will let you know if I found some results.
Regards,
Lucas.
Hi everyone,
it is possible to use dry electrodes for SSVEP. Hard to tell what's wrong here: the most common issue is that the hardware parameters are not correctly applied, as @retiutut mentioned it is sometimes tricky, especially if you stream the EEG flow to a third party software. The modifications of hardware parameters like gain, deactivation of a channel or bias are sometimes not correctly/totally implemented and validated in generic software even when Cyton/Ganglion are officially supported. @lucasbaldezzari is the signal you're looking at in the GUI the same signal that is processed ? Another thing that could possibly explain your problem, the default parameters that work for a SSVEP BCI with gel electrodes might not work directly when you swap electrodes to dry ones. Basically, dry electrodes tend to be a little more sensitive to noise if the skin is dry, if the pressure/contact is not right, if the hair are not combed away, if there is excessive EM noise from mains, etc... In comparison papers like the ones @wjcroft mentioned from some manufacturers, the comparison tests are usually performed in close to ideal conditions and there is basically no difference then between dry and gel (especially if the electrodes are pointy/rigid/metal/semi dry). If you are not in ideal conditions, you might get a little more noise and then you might have to adjust the SSVEP parameters, like maybe increasing the duration of acquisition before identifying the right frequency, adjust the filters. A preliminary step might be to assess the evoked potential response in the FFT first and figure out what parameters should be slightly adjusted. We got great results using C-VEP with ThinkPulse for spelling (using OpenBCI GUI and LSL) so I see no reason why you could not succeed with dry electrodes. Best, Julien
If the SSVEP peak in the FFT is obvious and strong, and your detection / classification algorithm / filter is not matching it, then it seems to imply some adjustment is needed in your detection criteria.
Regards, William
Hello everyone, how are you?
First of all, @julienConscious.
I hope so! But sometimes I think for some reason the signals perhaps are different, it has no sense because I'm using the Brainflow documentation and Open BCI documentation and I have configured the Ganglion or the Cyton boards in the same way that the GUI (the channels disconnection, gains) but perhaps I'm doing something wrong.
I did it what you mentioned, I increase the stimulation time, more LEDs for stimuli, changed the LEDs color, I use stimuli in a screen, I increase the number of averaged trials, but for dry electrodes, I haven't SSVEPs, but for gel cap, everything is fine. So, it is weird. It should be something of hardware configuration.
@wjcroft for your comment,
I will show you some pics.
I use this comment to show you some pics (I accidentally clicked "Post Comment", sorry.
All the experiments were performed using one Blue LEDs with a blinking frequency. The frequency was made using an Arduino with a square signal. I performed a 3 trial averaged an 4 seconds of stimulation for each trial and 2 seconds for rest time between trials. I only use 2 channels (O1 and O2). I applied a notch filter in 50Hz and a Bandpass Butterworth filter between 5 and 30Hz.
Next image shows the Fourier Spectrum for the 8.5Hz stimulus. You can see a 8.5 peak. This is for channel O1 only and gel cap electrodes.
Next image is the PSD for Welch method for the 9Hz Stimuli for O1 and O2 channels averaged and gel cap electrodes.
Next image is the Fourier spectrum for the 7Hz Stimuli for channel O1 only and gel cap electrodes. There are one peak at 7Hz and other in 14Hz (personally, I have noticed that at 7hz the first harmonic almost always exceeds the central frequency in amplitude, I think this is reported in the literature).
Finally, this is the Fourier spectrum for 7Hz stimuli but using Dry electrodes. This spectrum corresponds to the 7Hz Stimuli for channel O1 only. This corresponds for one of the three averaged trials, but if I show you any of the others averaged trials, the results are similar, I mean, the spectrum is very noisy and there aren't peaks either in the center frequency nor in the first harmonic.

I have a lot of more signals using gel cap electrodes and the system perform very well. I tried SVM, LDA, CNN, Logistic Regression like classification methods and all of them perform well when I use gel cap electrodes and the Fourier spectrom or the PSD from Welch's method like feature vectors.
All this work is because I am directing a BCI competition where my students will control a robotic vehicle through a race with obstacles using SSVEPs. The race will be the December 17. I will public the github repository when we finish, maybe it will be useful to you.
Regards,
Lucas
Hi Lucas,
thanks for images and for the explanations. Your experiment looks great and indeed you can see a very nice peak with the gel electrodes. I'm confident you can achieve similar results with the dry electrodes but it might require some adjustments. I do not know if this is the right place to discuss the SSVEP protocole here, and I'm not an expert in this but my first impressions for the dry electrode spectrum are 1/ your 7Hz peak is still here, with similar amplitude compared to previous spectrum and this is a local maximum. It looks like the 14Hz is here too with similar amplitude. The problem is more that other peaks are present with pretty high amplitudes too (9/10Hz, 19/20) and especially an extra 16Hz peak that is over 0.8 microV and higher than others, not present in the other spectrums. Is there anything around you that could explain this extra peak ? Since I believe your experiments were done in a similar environment with gel or dry electrodes, it looks like you are catching more EM noise with the dry electrodes hence the noisier spectrum. I would suggest to try to reproduce the experiment in a very non noisy location and also to make sure nothing around in your setup could produce some noise (computer, electronics, chargers, printer,...). Also it is always good to make sure you have a good contact of the sensors on the scalp, and also to try deactivating the potential other channels that do not show good contact/good signal, they could contaminate the good signal. Good luck with that and let us know how it goes !
Best,
Julien
What I don't understand about the posted graphs is the vertical scale. Is there a mistake? All these values are below 1 microvolt. EEG less than a microvolt is generally considered mostly noise. What FFT vertical scale peaks do you see when looking at this in the GUI? I would expect VEP peaks at least a few microvolts, probably more.
Hello guys, how are you?
Thank you @julienConscious. I will answer you.
Thanks for your comment, I will try to get the same results using dry electrodes.
Yeap, perhaps there are some SSVEPs information, but I'm not sure. I think it is only EEG activity.
Yes, you're right. But I will pay more attention and try again.
Great, I will do this.
Thank you @julienConscious, I will share the results with you as soon as possible.
Hello @wjcroft, how are you?
Yeap, it is weird. In the GUI app I can see more than 1uV. Please refer to the images below where you can see from left to right, the EEG for O1 and O2 averaged channels (filtered using a notch in 50Hz and a band pass between 5Hz-30Hz), O1 and O2. Then you can see the FFT and the PSD using the Welch method. Those images are for the 8.5Hz stimulus. Perhaps I'm implementing something wrong in my codification and that's the reason for very low amplitudes. What do you think?
Regards,
Lucas.
Hi Lucas,
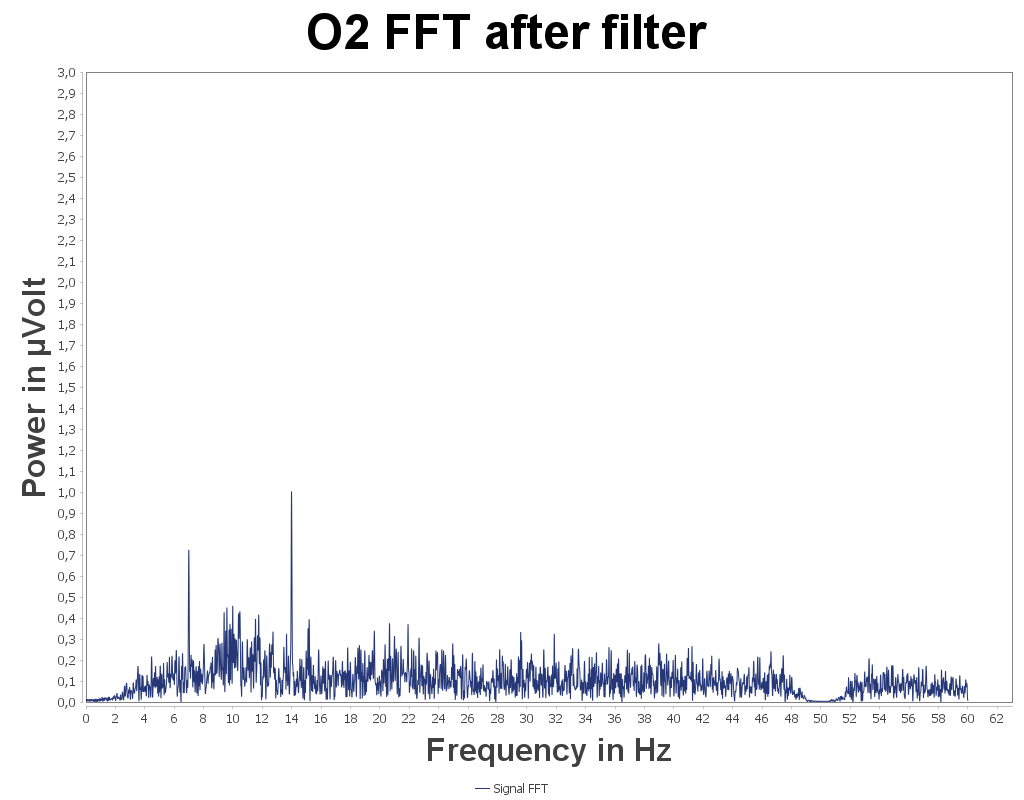

so I was curious, we made some quick experiments.
Here is the spectrum for O2 with a blinking at 7Hz:
and the spectrum still for 02 with a blinking at 11Hz:
Both recordings were done using the ThinkPulse active sensors over 20s (I did not try a shorter time), simple FFT, no Welch. We can see the expected peaks. What I noticed is how important it is to have a small 50Hz (or 60Hz) interference peak. It does not work well if the 50Hz interference is too high: dry sensors tend to catch more of this interference compared to wet sensors in the same conditions which could explain the problem in your experimentation. So I would suggest checking the unfiltered FFT in the OpenBCI GUI live and check the amplitude of the 50Hz peak, try to find a spot where it is as low as possible and do the experiment again. Keep us posted!
Best,
Julien
Hello @julienConscious, how are you? Thank you so much for your time, I appreciate it.
Your graphs look very nice. Luckily last friday my students made experiments, they controlled robotics vehicles using bci. For this, they use gel cap electrodes and gold cup electrodes -and Ganglion boards-. Everything was fine. I share with you a graph for the power spectral density using the gel cap -with three averaged trials- and some pics about the race.
Thank you for your support @julienConscious and @wjcroft. I will be around the forum next year, for sure!. Have a nice end of year.
Regards,
Lucas.
Hi Lucas,
Really cool experiment, it looks like a lot of fun ! Thanks for sharing.
Julien
Hi @wjcroft and @julienConscious,
I have recently purchased the ThinkPulse active electrodes and the OpenBCI Cyton + Ultracortex M4 and am trying to run a P300 experiment on it. However, I can’t seem to detect any P300 signal when using these active electrodes, which is why I’m hoping I can get some advice here.
To provide some context, I set up the headset with 8 electrodes (Fp1, FP2, PO3, POz, PO4, O1, Oz, O2) and wired everything according to the ThinkPulse videos (with ear clips at SRB2 and BIAS). I’m using the OpenBCI GUI to send the data via the LSL Networking module. As a sanity check, I opened the OpenBCI GUI visualizer to inspect the timeseries data. Even without disconnecting the BIAS earclip or changing the hardware settings, the signals seemed fine – I could see eye blinks, teeth clenching, and alpha waves from eyes closed recordings (see screenshots below).
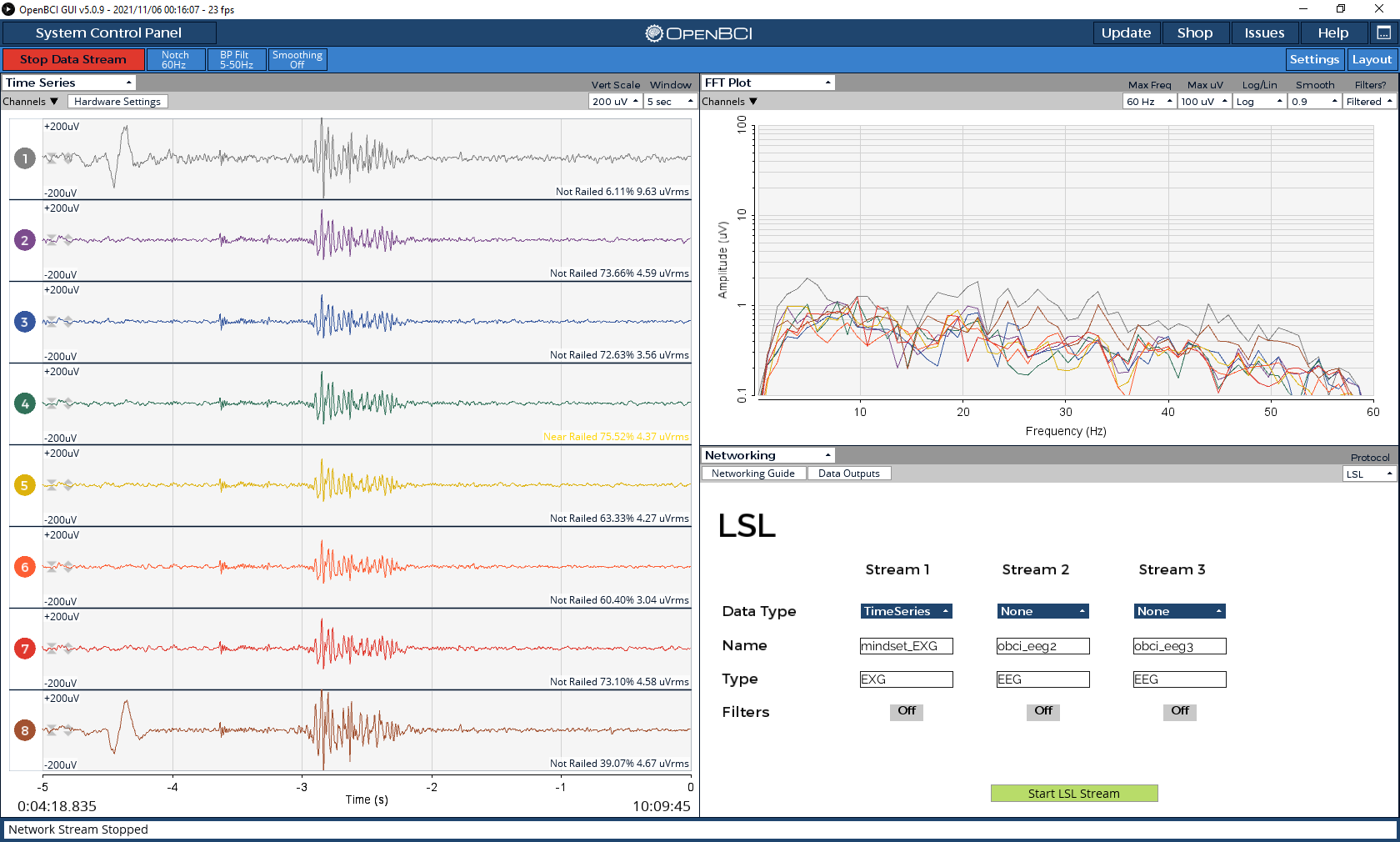
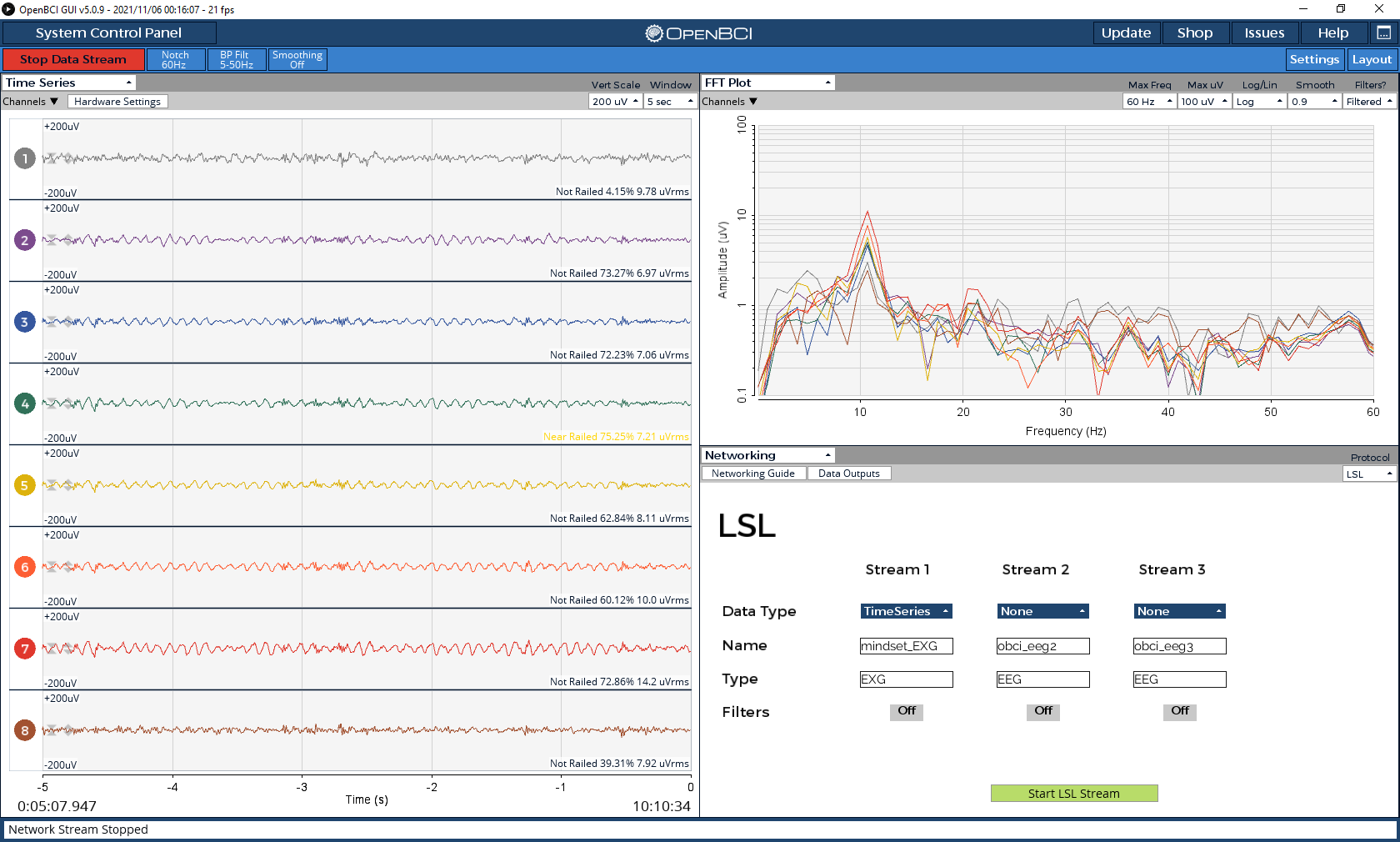
I then tried to collect data while doing a simple visual P300 experiment where I randomly flash a 4x4 grid. I collected around 15 minutes worth of data, which contains about 420 trials of flashing of the target button. To preprocess the data, I applied a bandpass filter from 1-25 Hz and resampled the signal to 50 Hz. I then averaged all the target trials to one grand average ERP (without the Fp1 and FP2 electrodes) to visualize the P300. However, I couldn’t see any noticeable ERP with the ThinkPulse electrodes. To make sure it wasn’t a problem with the experiment setup itself, I repeated the exact same experiment using a research-grade wet EEG cap (gel), and the P300 signal was very clear with an amplitude of 20 uV (see screenshots below).
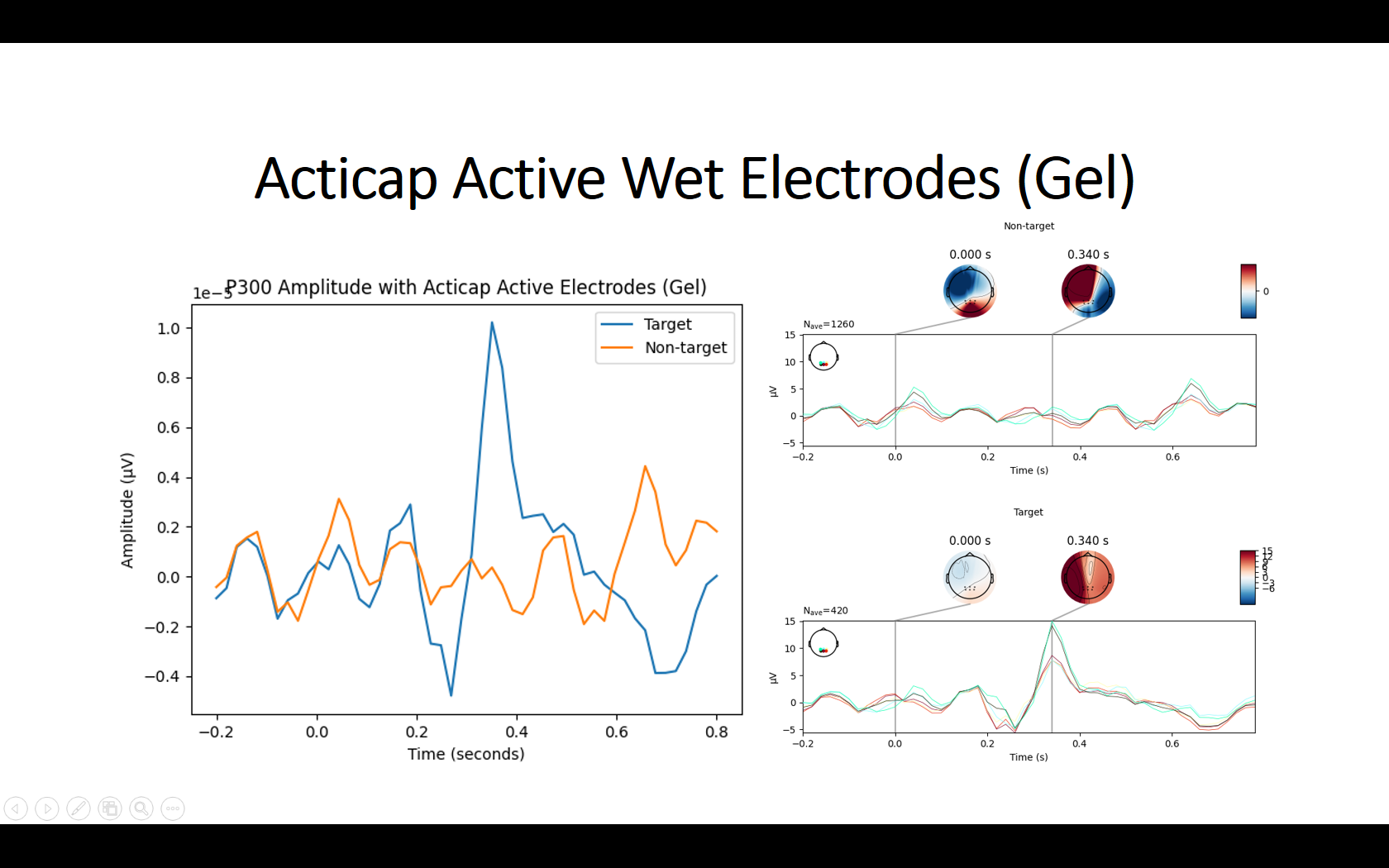
To rule out it was an issue with not having enough trials, I tried averaging only 3 minutes worth of data for the wet EEG cap (only 84 trials) and I still saw a P300 with an amplitude of 20 uV. Even though dry systems are noisier, it seems unlikely that I couldn’t notice the signal after averaging 377 trials using the ThinkPulse electrodes.
I was told that Conscious Labs had success measuring the P300 wave with the ThinkPulse active electrodes, so I’m confused why I’m not getting similar results. I’m not sure what could be going wrong here and would appreciate your help. I really hope I can get these electrodes to work. If they do, I definitely plan on buying more sets of them. Sorry for the long post, but please let me know if there are other details I can provide to help clarify things.
Thanks,
Jason
Hi @Jason,
thanks for your message and the detailed description of your experiment. We indeed have successful P300 experiments protocoles with ThinkPulse, sorry to hear it did not work for you. What you did for verification makes complete sense. Hard to tell what goes wrong when you use the ThinkPulse. It looks indeed like you have a very clean physiological EEG signal with the ThinkPulse setup. Even if the recordings were a bit noisier, the high number of repetitions of the stimuli should definitely increase the signal over noise ratio and show a P300 wave. A few questions:
Best,
Julien
Sorry for my English and sorry .. for the question, if possible!
About 18 months ago in this forum I posed the problem of ThinkPulse sensors+Ultracortex Mark IV + Cyton not working while the Cap with wet electrodes + Cyton worked very well (BCI P300 speller application).
In the last few days I was about to try the system again (ThinkPulse sensors+Ultracortex Mark IV + Cyton), but first I wanted to have a look on the Forum to see if there was anything new. Well, over the past 18 months, I have read more reports of malfunctions (both: SSVEP and P300).
(one: https://openbci.com/forum/index.php?p=/discussion/comment/17679#Comment_17679)
I thought: perhaps because there are only reports of problems on the Forum.
So I had an idea: is it possible to ask if there are any Openbci users who are working well with P300 speller applications and with ThinkPulse sensors+Ultracortex Mark IV + Cyton?
At the moment I read that only JulienConscius, by Paris, says that ThinkPulse sensors work well ... . He produces them... . (and I don't think he works with Ultracortex Mark IV + Cyton).
Also Retiutut wrote that he was working "great" (dicembre 2021). But is it so? I dont'read any about his work in 2022.
My question is: is it possible to ask if are there Openbci users who work well with ThinkPulse sensors+Ultracortex Mark IV + Cyton?
At the moment, it is as if we have a dozen ratings for a product sold on Amazon, all at a minimum.
I hope that Openbci will sell wet electrodes of the same quality as its other products (Cyton, cap with dry electrodes, etc...) in the near future.
Thank you!
Etc
Hi @etc,
I merged your new thread into this existing thread on the same subject. It is better to group related thread subject areas. That way, previous commenters see your new comment via email, because they are 'subscribed' to the thread. In particular, @julienConscious, the ThinkPulse developer will see your comment. Thanks.
In looking through this thread right now, I do not see your past comment on Thinkpulse. However I do see that you had a comment on the saline electrode cap, here:
https://openbci.com/forum/index.php?p=/discussion/3436/saline-based-electrodes-gelfree-bci-electrode-cap-kit#latest
This is a list of all your previous comments and discussions:
https://openbci.com/forum/index.php?p=/profile/comments/etc
I believe Julien will post a followup comment.
William
Hi everyone,
thanks William as usual for organizing the discussions in the forum and for including the people that might be interested in participating in the discussion.
Thanks Etc for your interest in BCI and for spending time testing our tech. Recording P300 waves can be tricky with TP+UltraCortex Mark IV+Cyton. I'm happy to help if you want to share results here or using DM: we can organize a call and see what's wrong (and post results in the forum later to help others). Good luck with your BCI experiments and I sincerely hope you will succeed in reaching your goals.
Best,
Julien
Hi everyone,
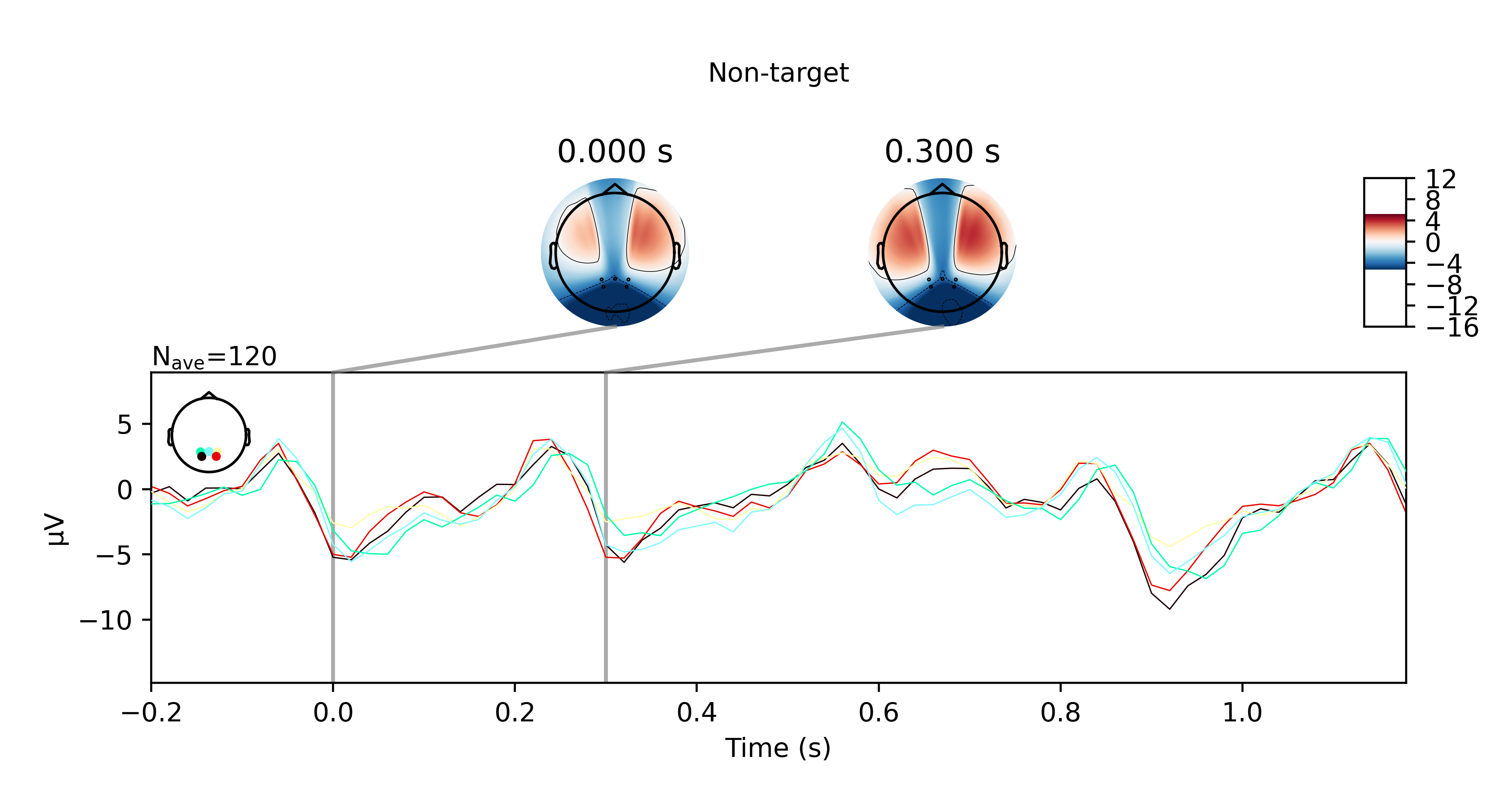
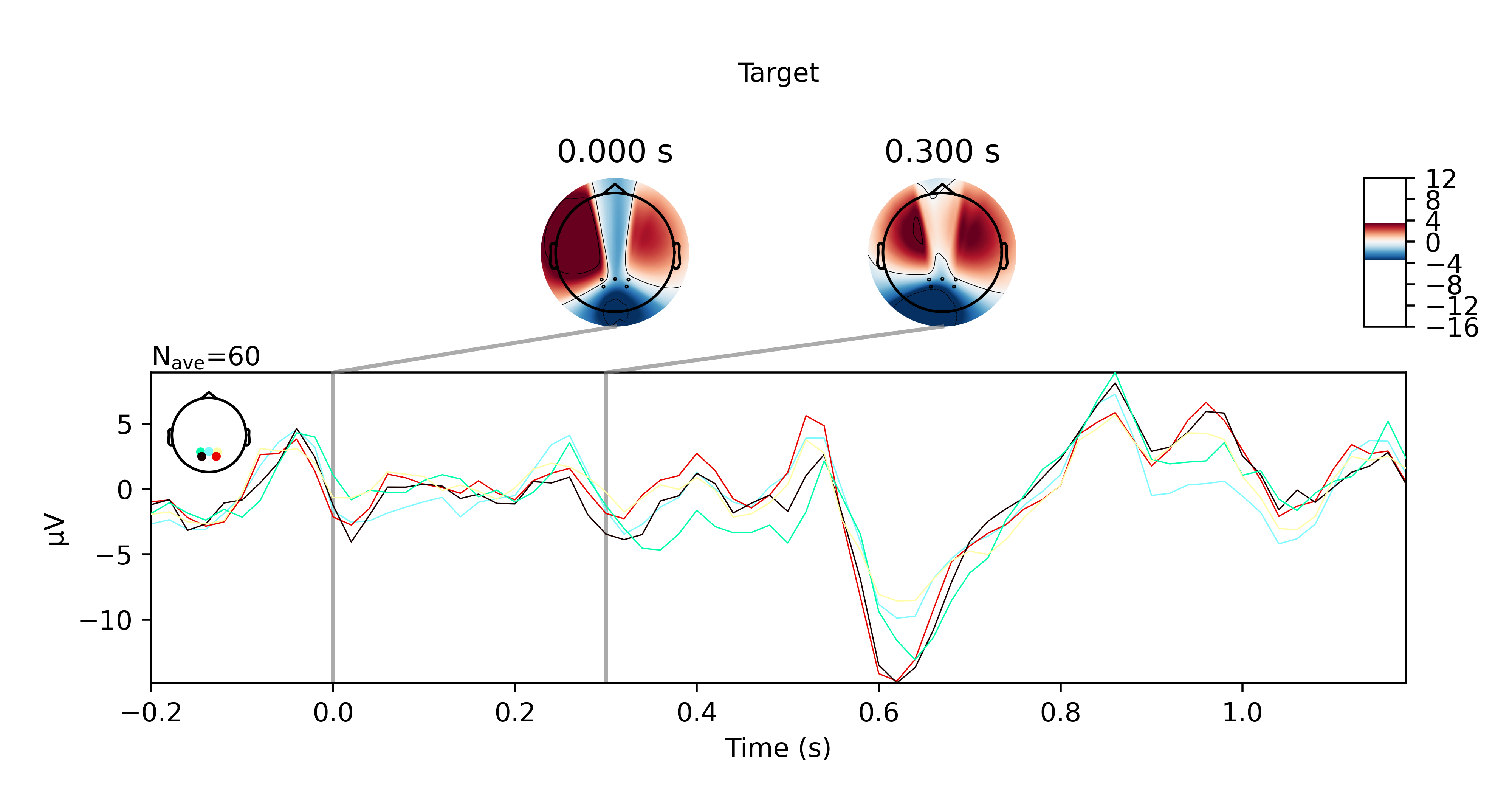
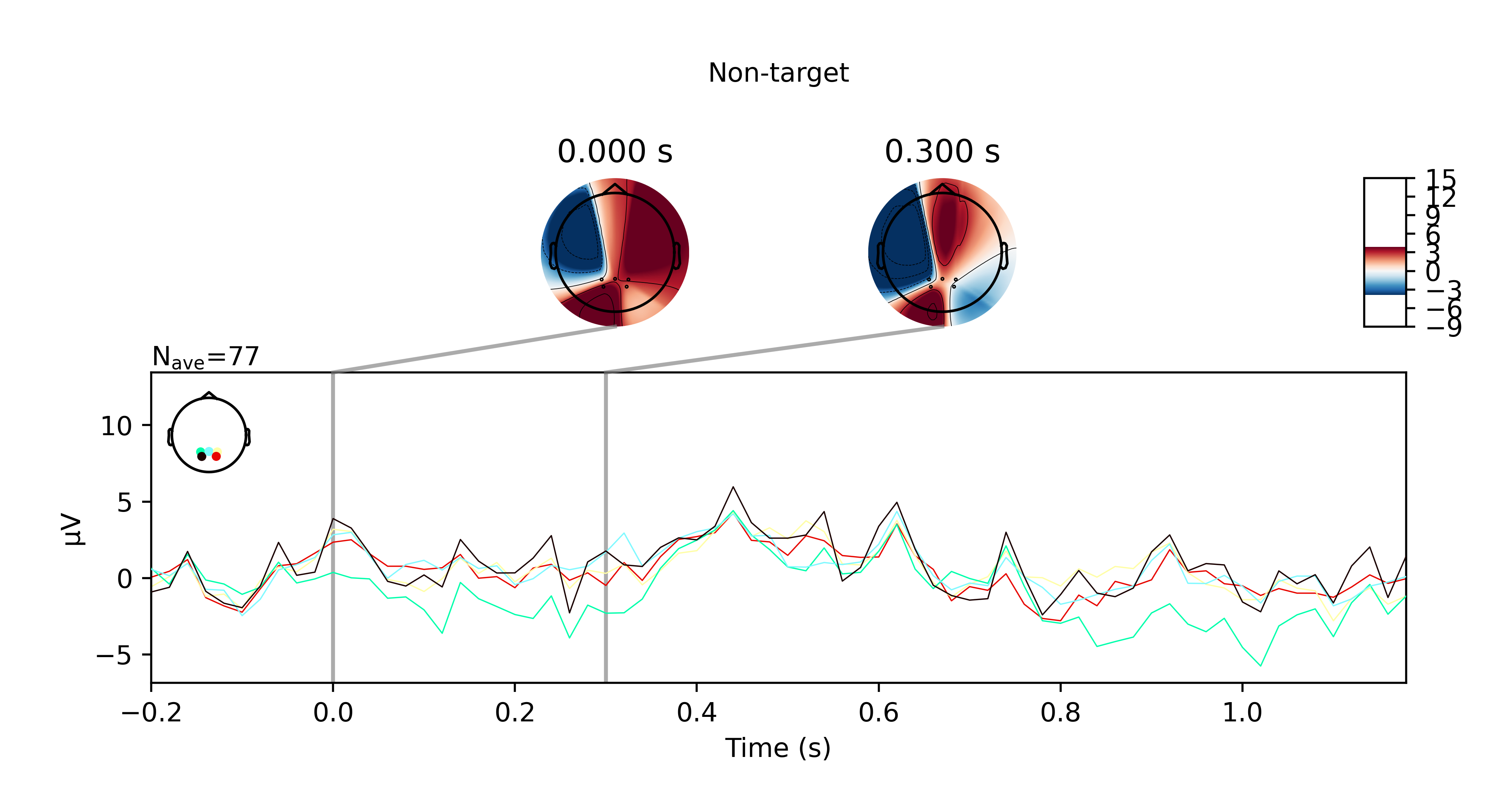
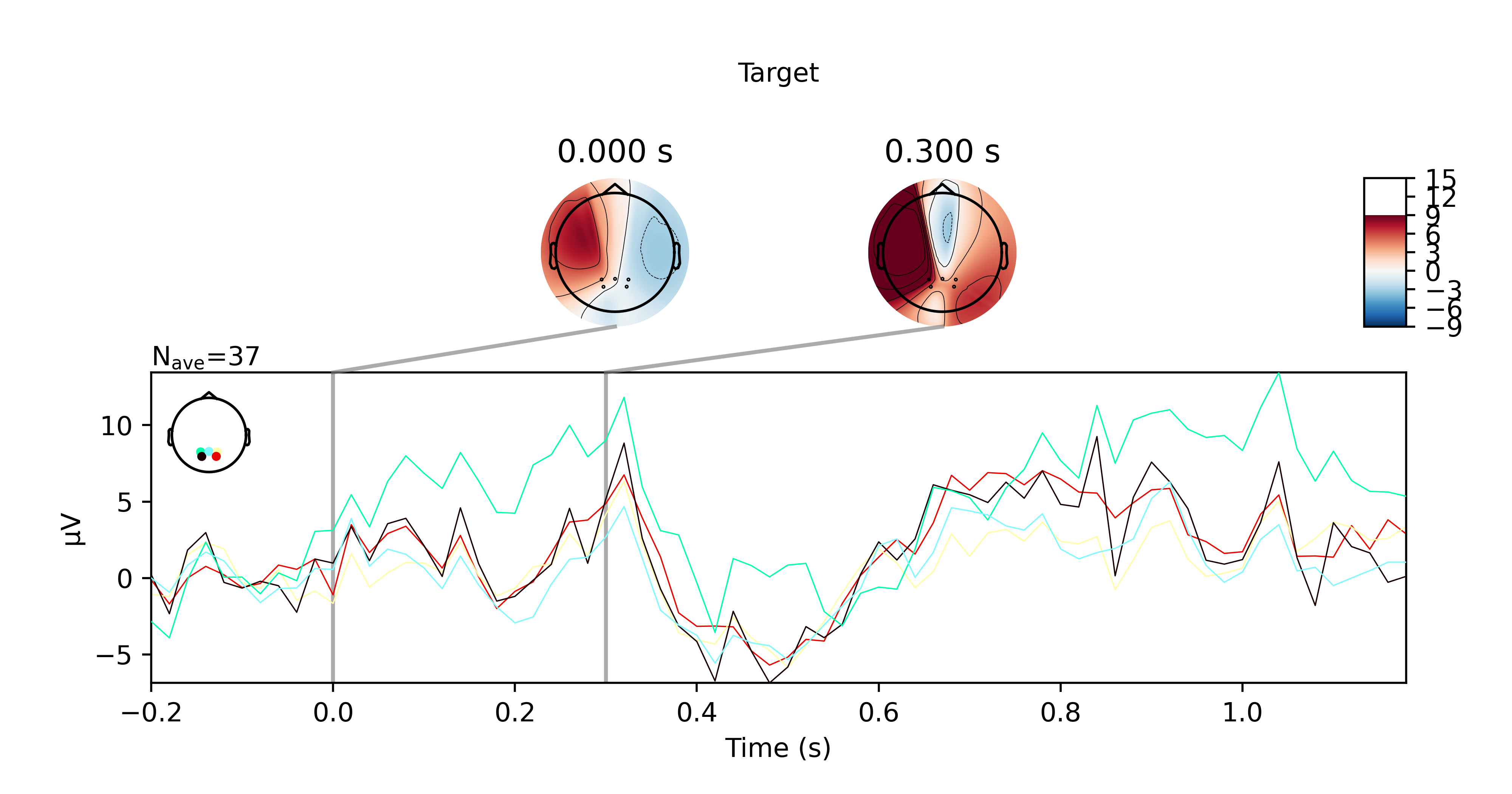
I got the P300 paradigm to work with the TP electrodes after all, for both offline and online trials. Here are some plots if you're interested. They show the averaged ERP response for nontarget and target trials for the offline and online trials respectively.
While this did work, I want to note that the sensors pick up noise very easily. I had to keep my head very still when doing this and even so, I can see high frequency bursts being picked up by the sensors every 10 seconds or so. Regardless, it didn't affect my results presumably because the bandpass filter I applied suppressed these artefacts.
Another disclaimer, this also isn't reliable for me 100% of the time. Sometimes I would not see any significant P300 response at all when I'm doing this in another room for example. I wonder if that has something to do the WiFi not being great there (since the OpenBCI data is transmitted over WiFi).
Anyhow, just wanted to share my results with the community. Please feel free to reach out to me if you have questions.
Thanks,
Jason
(Sorry for my very bad English)
@wjcroft, Dear Williams,
thank you for your organizing the discussion! (I wrote some comments about ThinkPulse in this forum inside a discussion about “issues with my 2 Cyton dongles / speller apps [resolved]))";
@JulienConscious,
after reading about ten post in these months with ONLY description of problems by users of TP+UltraCortex Mark IV+Cyton, I began to think that there are problems in this solution (Tp+UltraCortex Mark IV+Cyton). Before to try again to work with Tp+UltraCortex Mark IV+Cyton and before to spend a lot of time, I asked if is there an happy user of TP+UltraCortex Mark IV+Cyton. You in the past were very gentle to give suggestions to everybody: but, after your suggestions I didn't read about anyone that worked well
Now, I say thank you to @jason!
I read, after my question, for the first time that there is one happy (with some problems...) user!
Now I would ask to Jason what paremeters did he use; Have you some other suggestions?
I hope that there are other happy users of TP + UltraCortex Mark IV+Cyton to share the results.
In this manner we can read not only problems but also solutions!
Etc
ps Remember: my English is bad...
Hi @etc,
I just used 5 TP electrodes (PO3, POz, PO4, O1, O2) with reference (TP electrode) at Fz and ground (OpenBCI passive flat electrode) at Fpz. Julien actually recommended that the ground electrode should be a passive electrode, while the reference can be an active one. In the OpenBCI GUI, I kept all the hardware settings at the default, except I needed to turn off all the channels that are not being used (by setting "Input Type" to "Shorted"). Other than that, there were no other special hardware settings I needed to tweak.
Hope that helps.
Jason
Thank you, @jason!
The gain for every electrode was the same of default or less than 8?
Thank you!
I just left them as the default. Julien mentioned that you could lower them if the amplitude of the signal is too high, but leaving it on the default settings worked for me.
Thank you!
Last question: what tipe of electrode-Openbci did you use for the ground at Fpz? One of the electrodes of UltraCortex Mark IV?
Thank you very much! You were very gentle!!!
I just used the passive flat ones that came with the kit since I'm putting it at the forehead with no hair. I suspect the dry comb electrodes would work too. The ground electrode just needs to be passive I believe.
hi, I'm Anto , I tested THINKPULSE™ ACTIVE ELECTRODE KIT (Active Dry Electrodes) with our EEG acquisition System , but it's not Picking Signals if I connect Directly on Subject .
I supplied power supply of about +2.5V and -2.5. If I Give 100uV Sine Wave then its Working . And For Ground and Reference electrode I used Gel PAD Electrodes (Surface mount Electrode) and For Active I connected the THINKPULSE™ ACTIVE ELECTRODE via Gel Pad (Gel Pad Electrode is connected on Subject, From Gel pad to Spikes of Dry electrode I Used Crocodile Clip Connectors) then Its Picking the Signal(But only with Gel PAD instead of Directly placed on Subject Head).
I think Circuit Connection and Power Supply , All are okay because Its picking 100uV Sine Wave and Bio-signals only via Gel PAD but only not working if I connect directly on Subject .
Can Any one Help me to Solve this . Thank You.
Hi @anto,
I merged your new thread into this existing thread on Thinkpulse. Since they were designed for Cyton, I'm not clear that they would work with other "EEG acquisition systems". @julienConscious would know better.
Note the previous comments in this thread, the reference can be either ACTIVE or PASSIVE. You are currently using a passive gel electrode for the reference, you might try as active.
But more importantly note that your below comment, does not appear to be 'compatible' with normal Thinkpulse instructions:
The Thinkpulse conductive rubber comb should be in direct contact with the skin. There is no need to connect these combs to gel electrodes, in fact that may confuse the active electronics, most likely. Not sure why you are doing this, the whole idea of Thinkpulse is that it is a dry system. It's fine to connect Ground (or Reference) to a gel electrode.
William